The Centers for Disease Control and Prevention (CDC) revealed on Friday that the new KP.3 variant has surged to account for a quarter of all new COVID-19 cases across the nation, now assuming the status of the predominant strain of the virus.

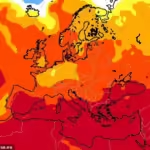
The rise of KP.3 coincides with an uptick in key indicators of virus transmission, a trend that the CDC has observed in recent weeks. Historically, previous years have witnessed peaks in virus surges around August.
Data obtained from the CDC’s surveillance of wastewater has shown an acceleration in virus levels particularly in the Western region. Concurrently, visits to emergency rooms due to COVID-19 have marginally increased across all age groups in recent weeks. The CDC currently estimates that COVID-19 infections are likely increasing in approximately 30 states and territories.
Comparative analysis indicates that KP.3 is now surpassing the prevalence of the KP.2 variant, often referred to as the “FLiRT” strain, which has recently risen to constitute 22.5% of cases. Although KP.2 had dominated in preceding weeks, its rate of growth has now decelerated.
Both KP.3 and KP.2 bear a striking resemblance to the JN.1 variant, which dominated the wave of infections during the previous winter season.
Natalie Thornburg, the chief lab official at the CDC’s Coronavirus and Other Respiratory Viruses Division, remarked on the remarkable similarity between KP.2 and KP.3 during a Food and Drug Administration meeting discussing the selection of strains for the upcoming fall vaccines.
While KP.2 and KP.3 exhibit minor discrepancies, early data suggests that mutations in KP.3 may possess a greater capacity to evade immunity compared to its counterpart.
The FDA’s announcement on Friday declared a decision to update fall vaccines to target the JN.1 variant, which had been predominant earlier in the year, thus forgoing the formulation aimed at the KP.2 variant.
Although Moderna presented animal study data indicating comparable protection offered by its KP.2-targeted vaccine against the latest variants, Pfizer’s vaccine for KP.2 demonstrated enhanced antibody responses against JN.1 variants, including KP.3.
In light of these developments, the FDA opted against KP.2 shots, as concerns arose among the agency’s advisers regarding its efficacy in broadening immunity against future strains compared to JN.1.